The Nitrogen Cycle and its Processes
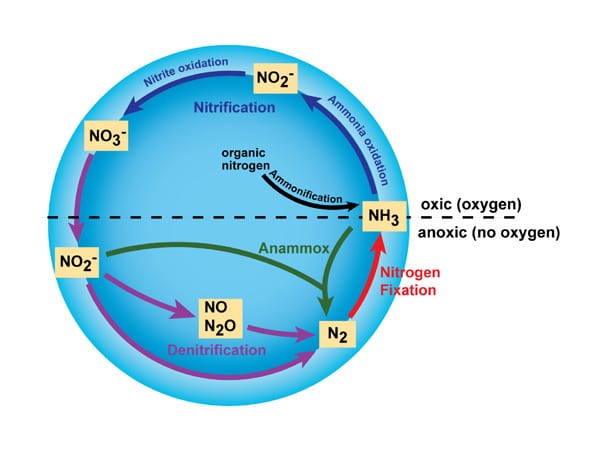
The nitrogen cycle explains the how nitrogen flows between animals, bacteria, plants, the atmosphere, and the soil on earth. The uniqueness of the nitrogen cycle is that nitrogen is the most abundant gas in the earth’s atmosphere, about 78% of all air, but it can’t be directly utilized by the animals and plants unless it is converted into usable compounds.
Its importance is because of its key role in the formation of nucleic and amino acids. It is also an essential part of adenosine triphosphate (ATP), which is the chief energy molecule for living things. For nitrogen to be used by plants and animals, it has to change into various states through the nitrogen cycle.
The major changes in the nitrogen cycle include nitrogen fixation, nitrification, assimilation, ammonification, and denitrification. These changes to different nitrogen oxides are dependent on various activities of microorganisms such as bacteria and fungi.
Processes of the Nitrogen Cycle
- Nitrogen Fixation
Nitrogen fixation is the process of converting the atmospheric nitrogen (N2) into biological state nitrogen. It is the first process of making nitrogen available for plants. It is defined as an anaerobic (without oxygen) process that catalyzes the reduction of atmospheric nitrogen (N2) into ammonia (NH3).
The process is solely carried out by prokaryotes (bacteria) which have the natural strength to break the triple bond between the nitrogen atoms. These nitrogen-fixing organisms are free-living bacteria whereas others are symbiotic nitrogen fixers. An example of a nitrogen fixer is the Rhizobium bacteria in the roots of legumes (soybeans, peas or clovers).
Other types of nitrogen-fixing prokaryotes are extensively distributed in different environments including terrestrial and aquatic settings. A special enzyme known as dinitrogenase is responsible for the fixation process. Once the nitrogen has been reduced to ammonia, the plants can now use it to make other biological compounds through the synthesis of enzymes, nucleic acids, chlorophyll, and proteins.
- Nitrification
Nitrification is the process where the ammonium ions (NH4) are converted into nitrides, first into nitrites (NO2–) then into nitrate (NO3–). Still, this process is done by the nitrogen-fixing bacteria. The first step is the oxidation of ammonia to nitrate, done by microbes termed as ammonia-oxidizers.
The second step is the oxidation of nitrite (NO2–) to nitrate (NO3–). The participating bacteria here are termed as nitrogen-oxidizing bacteria and they include nitrococcus, nitrobacters, and nitrosomonas.
- Assimilation
Assimilation refers to how plants and animals obtain nitrogen. Plant roots absorb nitrates from the soil into the roots then into the entire plant system. The plants then use the nitrates in the synthesis of nucleic acids, enzymes, amino acids, proteins, and chlorophyll. On the other hand, animals assimilate nitrogen by eating the plants.
- Ammonification
Ammonification is also termed as the decaying process. It occurs when the plant or animal dies then decomposers such as fungi and bacteria decompose the tissues and transforms the nitrogen back into ammonium. The ammonium then reenters the nitrogen cycle where it is taken up by plants and other microorganism for development. Animal waste equally releases ammonium into the nitrogen cycle.
- Denitrification
Denitrification is the process that changes nitrate to nitrogen gas, hence returning it into the atmosphere. This process releases the excess nitrogen in the soil back into the atmosphere. Special prokaryotes known as denitrifying bacteria carry out this process of reversing nitrates into nitrogen gas.
Unlike nitrification, this process is anaerobic (uses oxygen) and the bacteria involved are in the genus Pseudomonas, Paracoccus, and Bacillus. Dinitrogen gas (N2) is the final outcome of denitrification, but other nitrogen-related gaseous forms can as well be released. A good example is nitrous oxide (N2O) which is considered a potential greenhouse gas.






